Navigation auf uzh.ch
Navigation auf uzh.ch
We have a long-standing interest in the interplay between pathogens and host SUMOylation. SUMO proteins are small ubiquitin-like modifiers that can be reversibly attached to other proteins to alter their function, and play critical roles in cellular activities such as transcription, DNA damage responses, and immunity (reviewed in Everett, Boutell & Hale, Nature Reviews Microbiology, 2013).
Previously, we applied large-scale quantitative proteomic approaches to identify cellular and viral proteins that change in SUMOylation during virus infection, as well as during the innate immune response (Domingues et al., Cell Reports, 2015; Schmidt et al., PNAS, 2019). These resources have proved to be an important starting for many new projects in the laboratory, including those relating to influenza virus host-range and adaptation mechanisms (Domingues et al, Cell Reports, 2017;Domingues et al., Nature Communications, 2019).
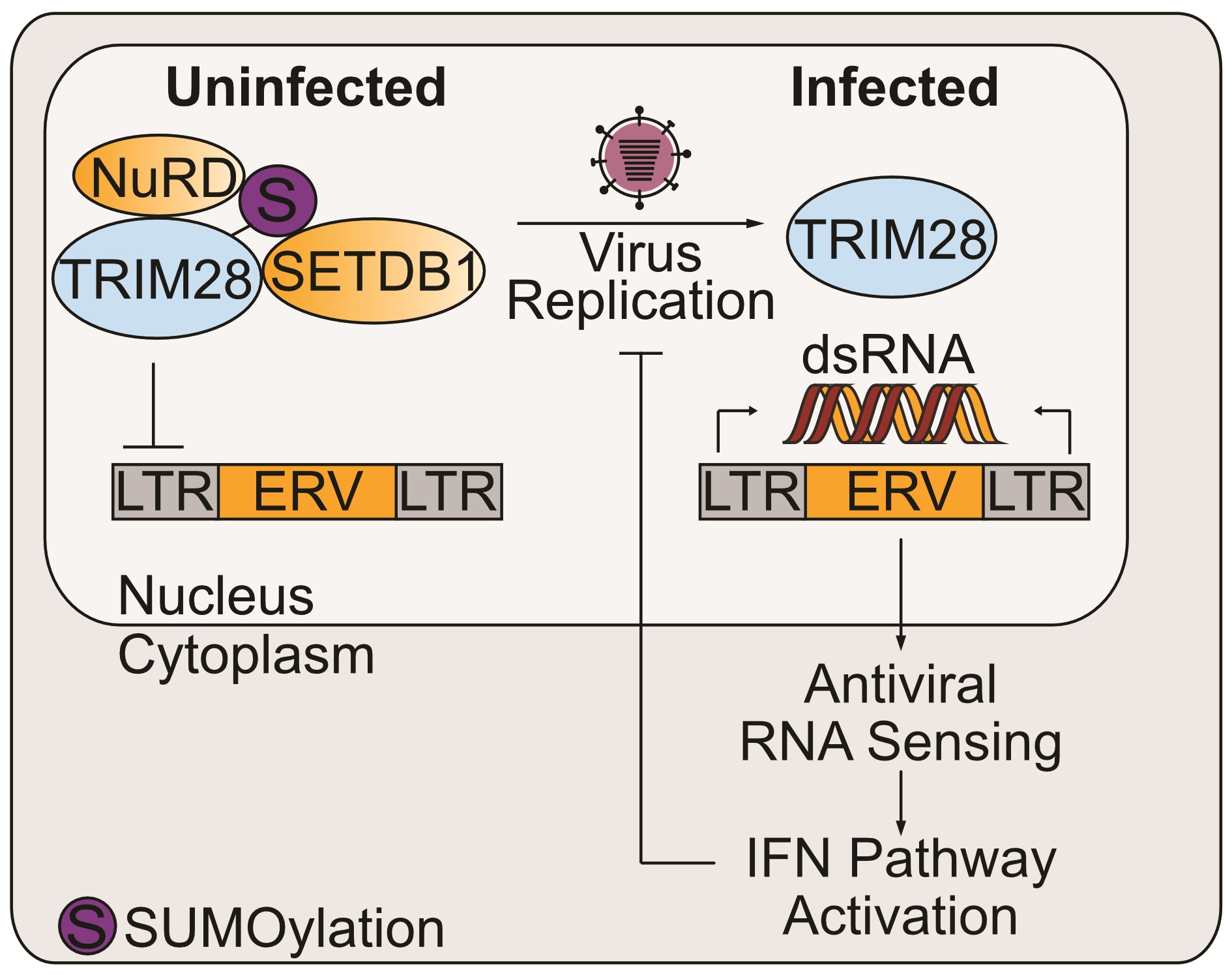
In one major follow-up, we detailed evidence to suggest that loss of SUMO-modified TRIM28/KAP1 is a previously undescribed host response to influenza virus infection leading to transcription of endogenous retroelements, which may form immunostimulatory ‘self’ dsRNAs and activate antiviral responses (Schmidt et al., PNAS, 2019). Such a mechanism would blur the canonical view that a host must strictly discriminate between ‘self’ and ‘non-self’ RNAs to defend against invading pathogens and prevent autoimmune reactions. We are now actively investigating how a virus-infected host may re-activate endogenous retroelements and co-opt them for antiviral defense. We are interested to identify the viral triggers and cellular machinery involved at the molecular level, and delineate how presence of potentially immunostimulatory ‘self’ RNAs can be detected by the host cell and modulated by certain pathogenic viruses (reviewed in Hale, Current Opinion in Virology, 2022).