‘Multi-omics’ of the Interferon System
Interferons (IFNs) are cytokines produced by infected cells that signal to neighboring tissues and induce an antiviral state to inhibit pathogen spread. As such, the IFN system constitutes a major innate immune barrier to zoonotic virus transmission and limits the severity of seasonal and pandemic disease. Recombinant IFN-based therapies could also be potent, host-directed, broad-spectrum measures for virus control and prevention in emergency situations (Busnadiego et al., mBio, 2020).
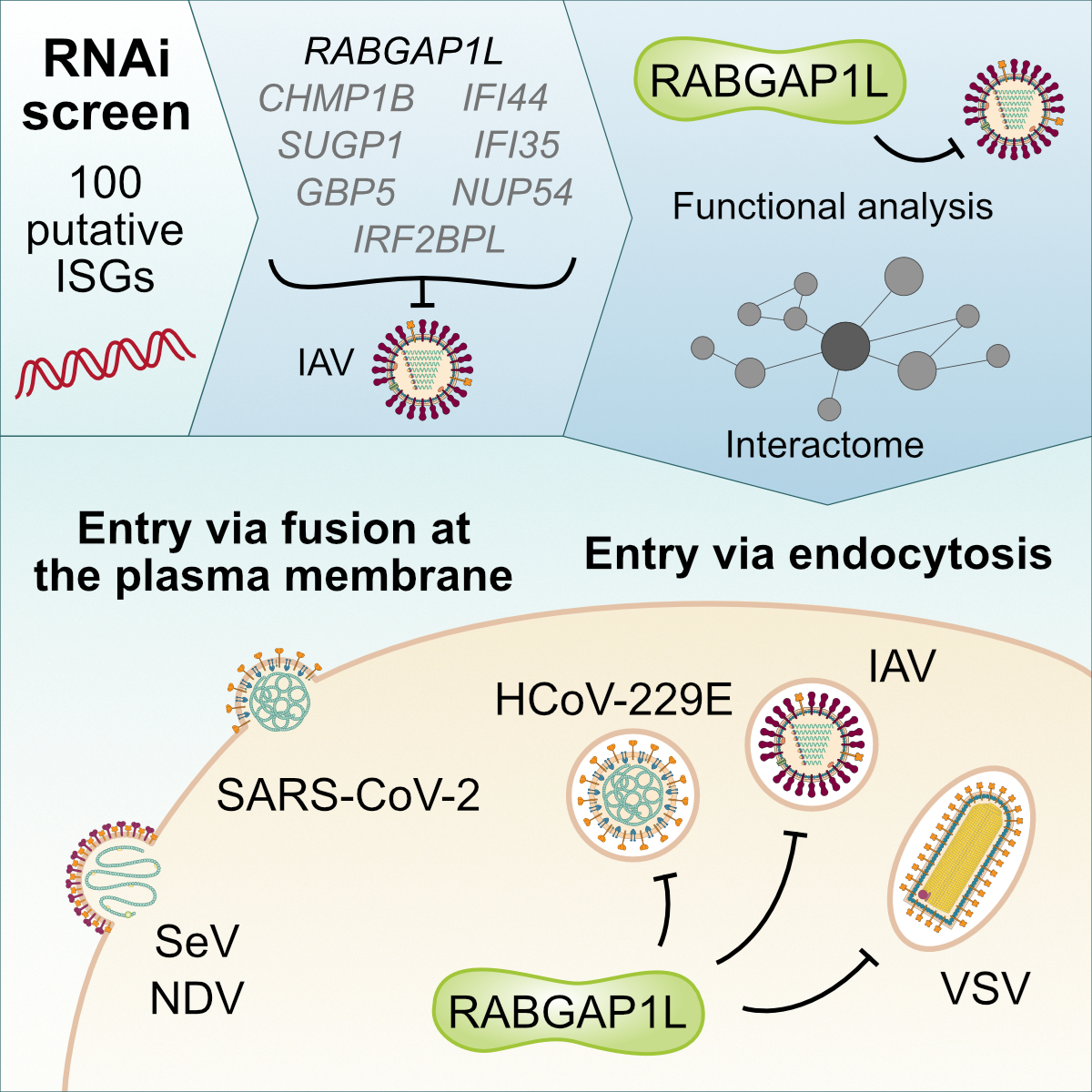
We are interested to understand more about the fundamental signaling mechanisms underlying regulation of the human IFN system. This is essential to fully exploit the concept of using IFN-based therapies, and to suggest optimal targets for new intervention strategies. Furthermore, this basic molecular research creates a knowledge framework to understand key factors that may be aberrantly regulated in infection-susceptible individuals with unknown IFN system deficiencies. We are currently adopting a ‘multi-omics’ approach to learn more about the IFN signaling pathway and the cellular processes that occur during mounting of the host antiviral response. For example, we have employed genome-scale CRISPR discovery approaches to identify new, druggable components of IFN signaling that could also be novel anti-inflammatory targets (Börold et al., EMBO Reports, 2021). In addition, we have performed restriction factor screening assays to identify new human proteins that mediate the antiviral action of IFN (Fernbach et al., Cell Reports, 2022). We have complemented these studies with proteome-wide mass spectrometry work to delineate and characterize important IFN-induced post-translational modifications (Busnadiego et al., PNAS, 2025), as well as state-of-the-art spatial and temporal protein interactome analyses to dissect IFN processes and regulation (Schiefer & Hale, Nature Communications, 2024). These efforts all illuminate the dynamic IFN-induced signaling landscapes of human cells, and will help to understand the mechanisms by which IFNs orchestrate host antiviral defenses.
Figure legend: Restriction factor screening identifies RABGAP1L-mediated disruption of endocytosis as a host antiviral defense (Fernbach et al., Cell Reports, 2022).