Navigation auf uzh.ch
Navigation auf uzh.ch

List of publications (ResearchID)
Our group focuses on two central topics: The HIV entry process and the humoral immune response elicited during HIV infection.
The HIV envelope (Env) protein trimer mediates attachment to and entry into target cells and is the only viral protein exposed on the surface of HIV. Neutralizing antibodies directed against the HIV Env complex can block virus infection. Most patients develop strain-specific antibodies against their own (autologous) virus, but elicitation of broadly neutralizing antibodies (bnAbs) able to neutralize heterologous primary viral isolates from different clades is relatively rare. A main focus of our research is dedicated to unraveling the processes that steer bnAb development in natural HIV-1 infection to guide HIV vaccine development. In this context we analyze factors that contribute to bnAb elicitation, explore the interplay of infecting virus and antibody development over the course of the infection, study the mechanisms and determinants of neutralization efficacy, and investigate the fate of B cells in HIV-1 infection. In a second line of research we use the knowledge on natural occurring bnAbs and the patient derived viral envelope (Env) proteins to develop Designed Ankyrin Repeat Protein (DARPin) based inhibitors of HIV-1 entry that mimic bnAbs in their activity. The combined knowledge on bnAbs, broadly neutralizing DARPins (BND), their epitopes and escape patterns is used in a third line of projects aiming to devise vaccine immunogens that evoke bnAb responses.
Only approximately 10-25% of HIV-1 infected patients develop potent bnAb activity. While patients do not profit from this bnAb response due to the very rapid mutation and escape of the autologous viruses, the excellent potency and breadth of bnAbs against viruses of different genetic subtypes make them attractive components of HIV therapy. The ultimate goal is however to create bnAb inducing vaccines. Despite decades of HIV vaccine development all strategies to induce potent neutralizing responses have failed so far.
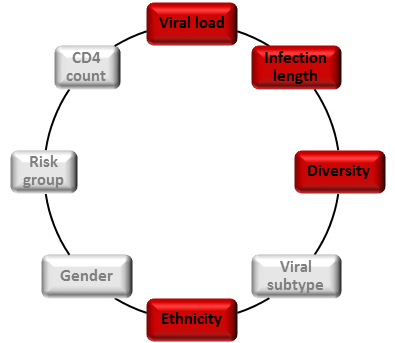
Therefore, understanding why only a fraction of patients develop bnAbs as well as determining factors that promote or restrict the development of bnAbs in HIV-1 infected individuals will be crucial for the design of successful vaccine strategies.
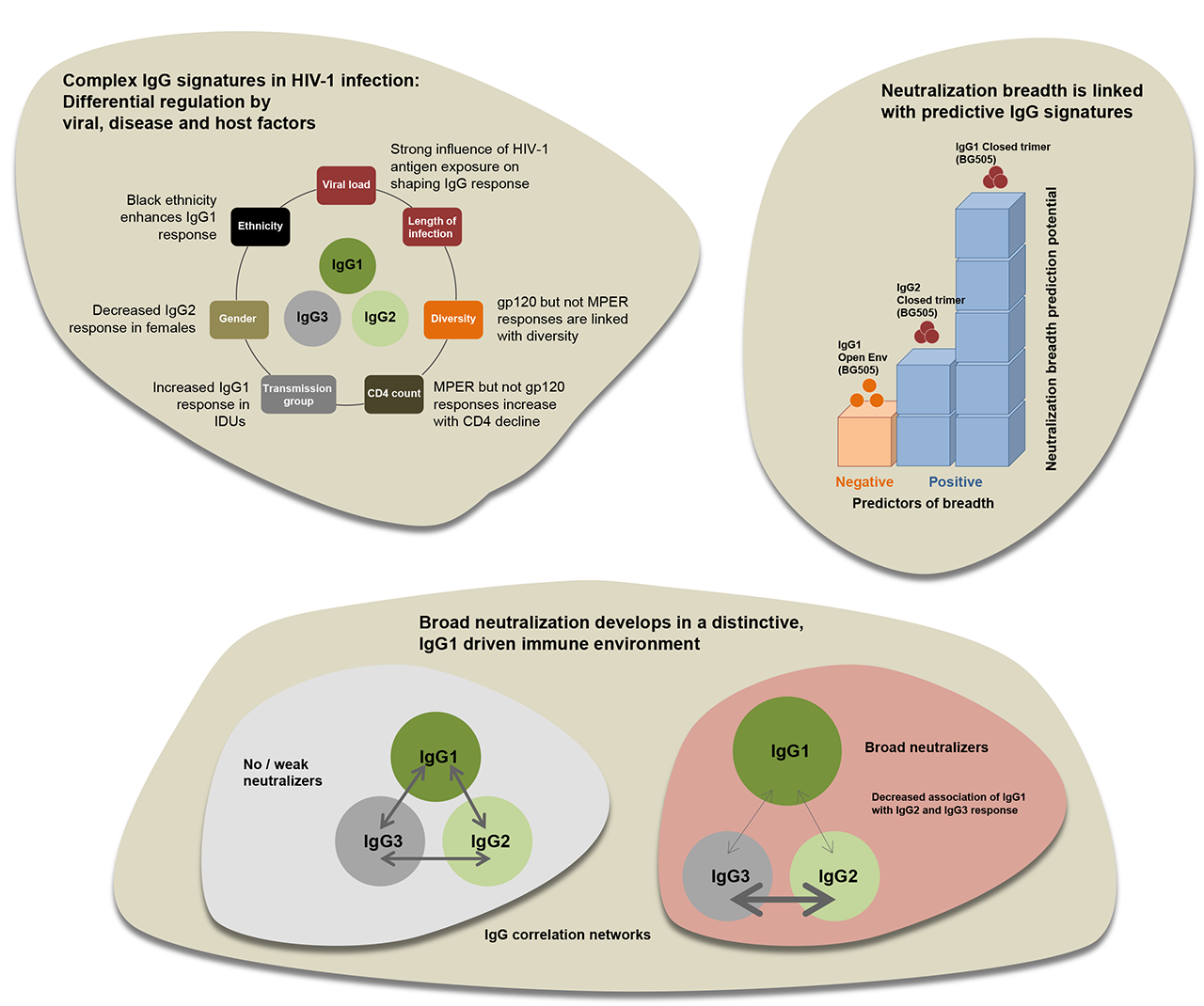
Making use of the extensive data and sample collection of two HIV cohorts, the Swiss HIV Cohort Study (SHCS) and the Zurich Primary HIV Infection Study (ZPHI), we conducted the Swiss 4.5K screen, a systematic survey to identify patients who develop bnAbs. In total, 4,484 HIV-1-infected individuals were screened for neutralization activity and viral, host and disease factors linked with neutralization breadth were investigated. We found that viral load, length of untreated infection and viral diversity correlated with bnAb induction. Further, individuals of black ethnicity were significantly more often found to develop bnAbs. The virus subtype did not have an influence on the frequency of bnAb induction but steered the specificity of the response.
Building on the results of the Swiss 4.5 K, we currently investigate immune signatures in our cohort involved with bnAb induction, the co-evolution of Env and Abs in bnAb inducers and the impact of the host and virus genome on shaping the bnAb responses.
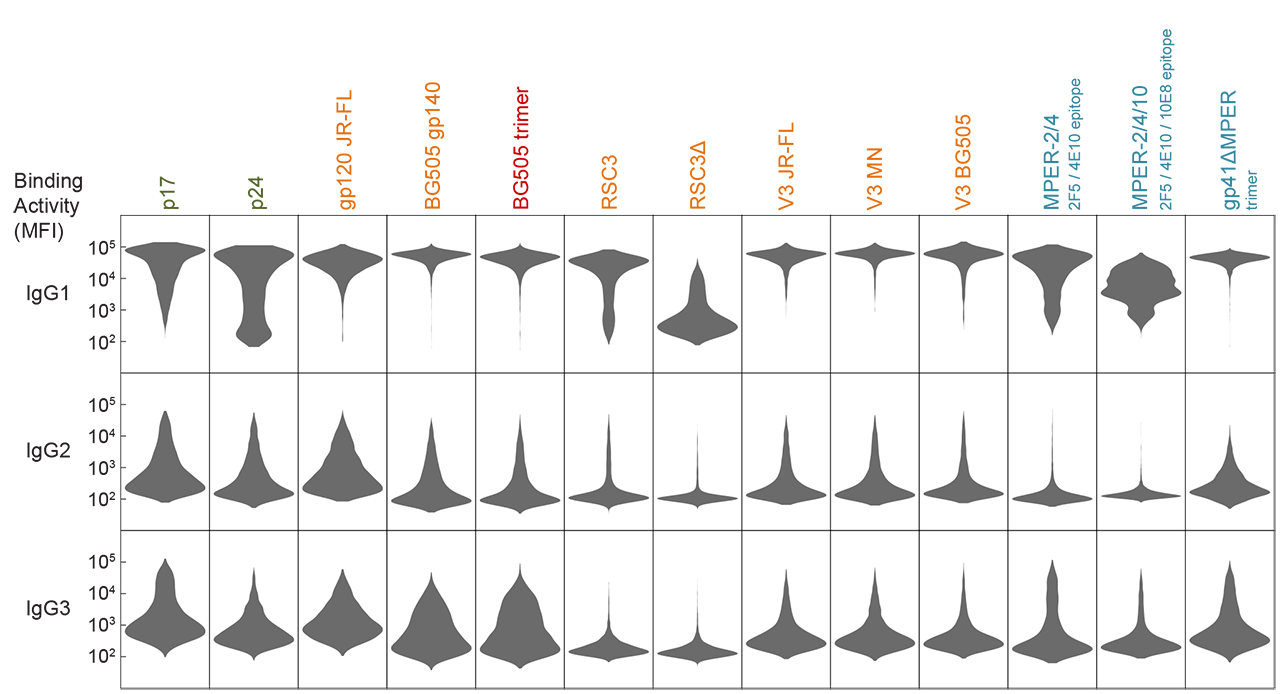
By establishing comprehensive antibody signatures based on IgG1, IgG2 and IgG3 activity to 13 HIV-1 antigens in 4,281 individuals in the same cohort, we showed that the same four parameters that are significantly linked with neutralization breadth, namely viral load, infection length, viral diversity and ethnicity, also strongly influence HIV-1 binding antibody responses. The effects proved however selective, shaping binding antibody responses in an antigen and IgG subclass dependent manner. IgG response landscapes in bnAb inducers indicated a differentially regulated, IgG1-driven HIV-1 antigen response and IgG1 binding of the BG505 SOSIP trimer proved the best predictor of HIV-1 neutralization breadth in plasma. Our findings emphasize the need to unravel immune modulators that underlie the differentially regulated IgG response in bnAb inducers to guide vaccine development.
References:
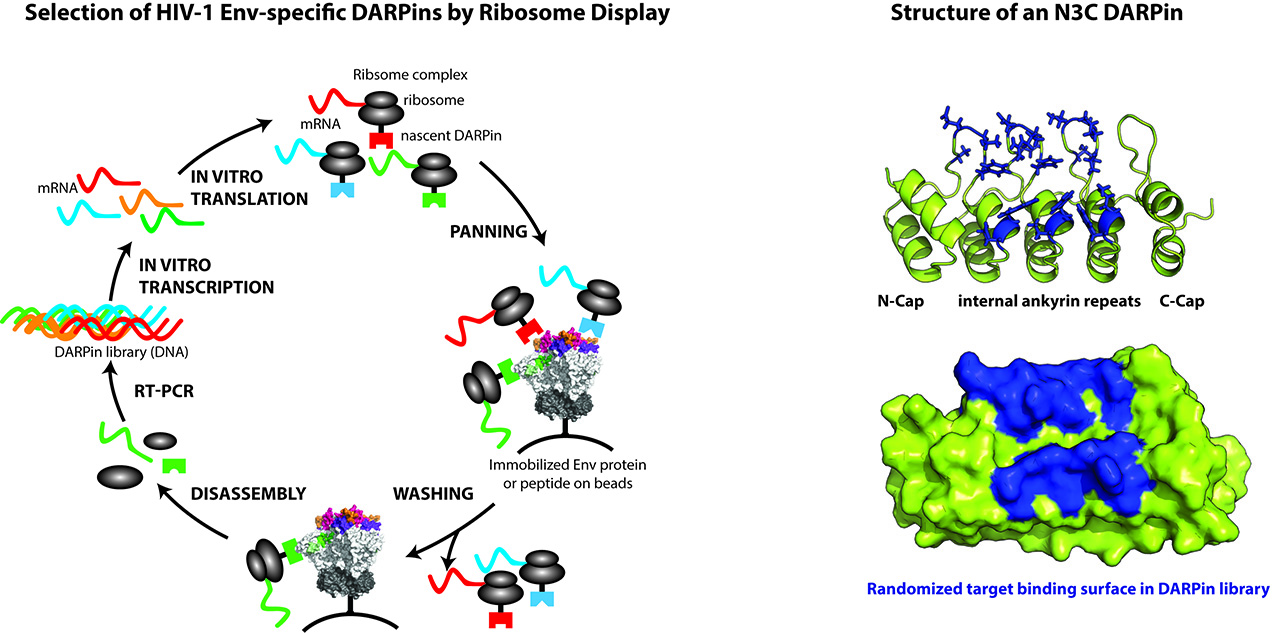
In this project area, we focus on isolating novel bnAbs and inhibitors that have the same broad neutralizing activity as the best bnAbs known to date. Studying the 239 bnAb inducing patients discovered in the Swiss 4.5K screen, we have a unique opportunity to discover novel potent bnAbs for HIV-1 therapies. Additionally, we focus on inhibitor development based on the Designed Ankyrin Repeat Protein (DARPin) technology. DARPins are attractive candidates for protein-based inhibition as they share high target specificity and affinity with antibodies while having a higher chemical and physical stability and displaying a different binding mode. Projects on Env specific DARPins are imbedded in two large collaborative projects: the European HIV Vaccine Alliance (EHVA; http://www.ehv-a.eu/) and the Gilead Award “SEEK, UNCOVER and ELIMINATE: Eliciting Antiviral and Infected Cell-Directed Activities Towards a Cure of HIV-1” (http://www.gilead.com/news/press-releases/2017/1/gilead-awards-more-than-22-million-in-grants-to-support-hiv-cure-research).
References:
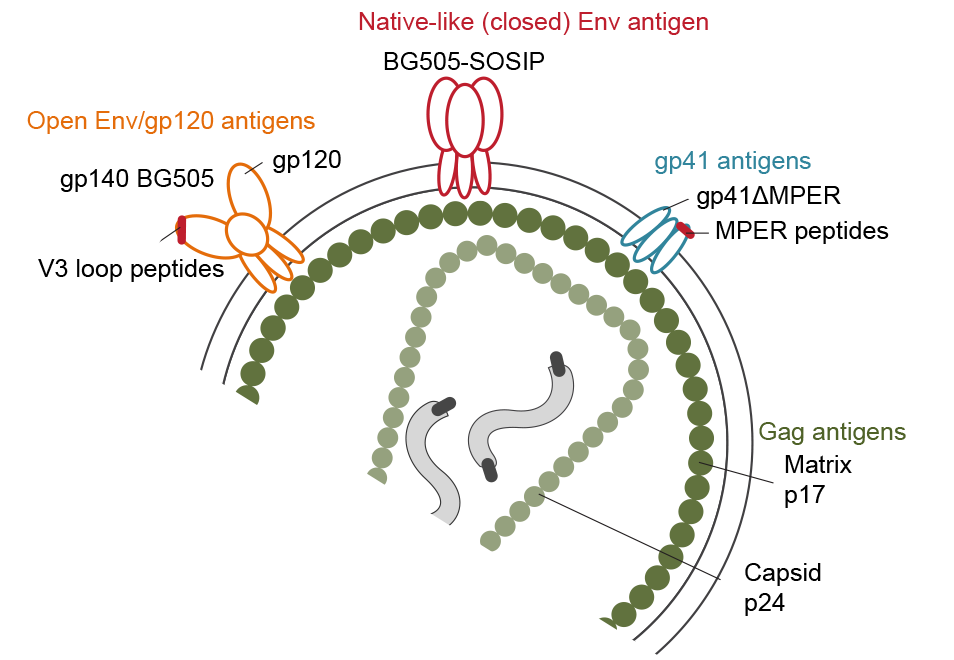
In this project area, we study the B cell response in HIV-1 infection in a wider context. We investigate B cell perturbations at different disease stages including successful antiretroviral treatment. We explore the B cell repertoire on a phenotypic and functional level using high-dimensional flow cytometry analysis, functional assays and Ig repertoire analysis. We further study both HIV specific antibody as well as antibody responses to other antigens to obtain a comprehensive picture of the humoral immune response in HIV-1infection. A particular focus of our studies is devoted on B cell responses in bnAb inducing individuals aiming to unravel B cell phenotypes and immune profiles linked with bnAb induction.
References:
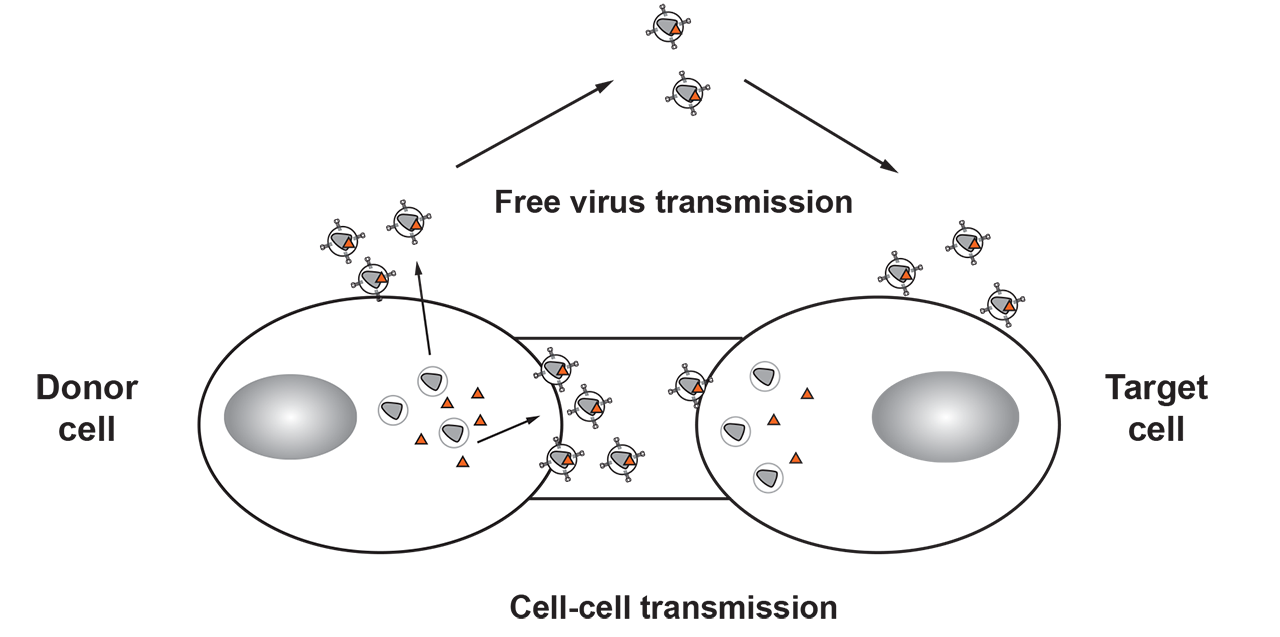
HIV-1 uses two distinct mechanisms to transmit between infected and non-infected target cells. While spread as free virus particles that bud from infected cells allows for the transmission to distant tissues and between hosts, the direct cell-cell transmission from infected to non-infected cells can help the virus to overcome physical and immunological barriers. BnAbs are considered vital components of novel therapeutics and blueprints for vaccine research. Yet escape to even the most potent of these antibodies is imminent in natural infection. Understanding why HIV-1 cell-cell transmission is more resistant to neutralizing antibodies and how this impacts escape to neutralizing antibodies and drugs in vivo is thus of high interest.
References:
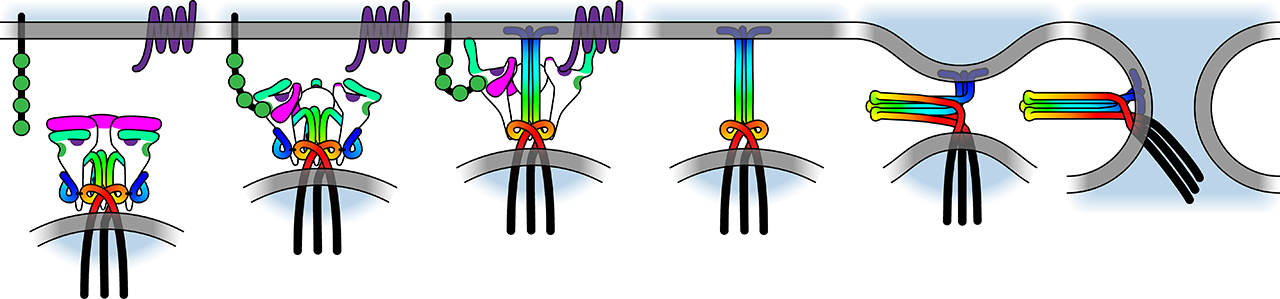
In this project area, we investigate molecular principles of HIV-1 entry and neutralization. We explore how many trimers are required to interact with target cell receptors to mediate virus entry, how many antibodies are required to neutralize a trimer and how this influences transmission of HIV-1. We further dissect the interaction of the HIV-1 envelope with CD4 investigating the structural conformations that are established upon CD4 binding and the adaptation of HIV-1 to cellular environments with low CD4 dependency.
References: